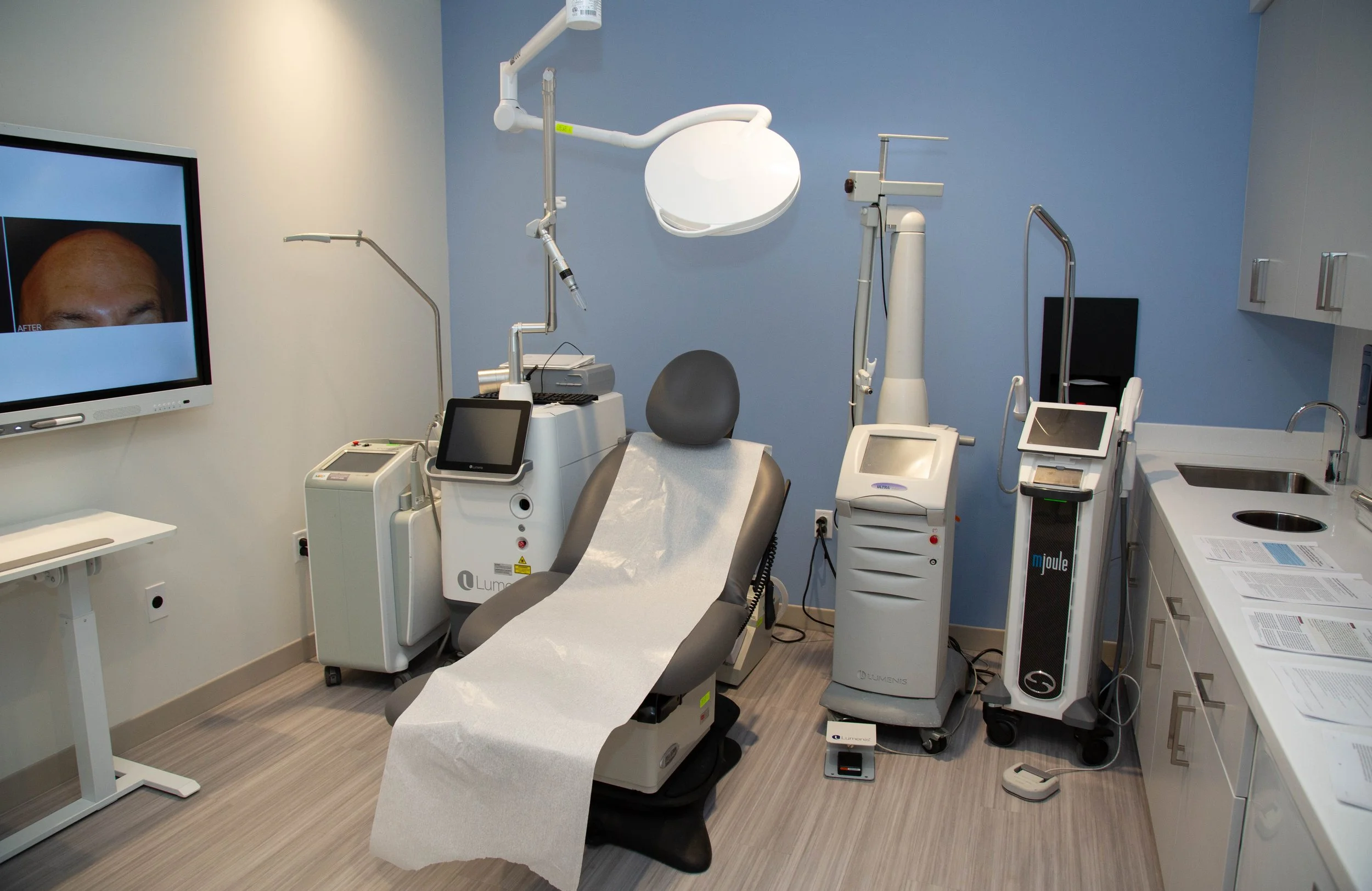
Clinical Trials Division:
Experience the future of aesthetic and medical dermatology with our expert team.
The following clinical trials are currently active and accepting patients at our location. If you are interested in becoming part of one of our listed clinical trials, please email our Clinical Trials Division at mdlr@miamidermlaser.com or fill out the form below.
-
We have various Atopic Dermatitis clinical trials currently enrolling.
Leave your information below and we may find a trial that is right for you.
-
You may qualify if you:
Are an adult (18 y/o +) with subacute or chronic cutaneous lupus (including discoid lupus), confirmed by skin biopsy
Have active skin lupus despite standard treatments
Have moderate to severe skin involvement
Test positive for at least one lupus-related autoantibody
What the study offers:
Access to investigational treatment for skin lupus
Study-related care and close monitoring by experienced clinicians
Opportunity to contribute to advancing lupus research
-
We have various Psoriasis clinical trials currently enrolling.
Male or female 40+ y/o
Have moderate to severe plaque psoriasis
Are a candidate for phototherapy or systemic treatment (including biologic therapy)
Have at least one cardiovascular risk factor, such as:
Smoking
High blood pressure
High cholesterol
Diabetes (type 1 or 2)
Obesity
Personal history of heart attack, stroke, or other heart procedures
Family history of early heart disease or sudden cardiac death
2.Palmoplantar Psoriasis
-
Evaluating a new oral treatment for adults with generalized (non-segmental) vitiligo.
You may be eligible for this study if you:
Are 18 to 74 years old
Have been diagnosed with generalized (non-segmental) vitiligo
Have vitiligo affecting more than 5% of your body surface area
Have at least one vitiligo patch larger than 2 cm² (not located on the face, hands, or feet)
Are willing to attend scheduled study visits
Are able to provide written informed consent
For women who are able to become pregnant:
A negative pregnancy test is required before starting
Effective contraception must be used during the study
-
-
Item description
-
-
This study is designed to assess the safety and effectiveness of dermal microcoring to improve:
Cheek facial volume loss
Skin laxity
Moderate facial wrinkles
*as a result of GLP-1 weight loss
Participants must:
Be 22+ y/o
Male or female
Have a history of GLP-1 medication use
Experience facial volume loss or wrinkles, particularly in the cheek area
Meet additional study eligibility criteria (determined during screening)
-
-
Registration:
Please fill out the information below and if eligible, we will be in contact.
Miami Dermatology and Laser Research
The board-certified dermatologists of Miami Dermatology & Laser Research are heavily engaged in clinical research trials, teaching, writing and editing journal articles and textbook chapters.
Jill S. Waibel, MD, FAAD has been an instructor and teaching medical students, residents and fellows and directed educational sessions and programs at national and international meetings, including the American Academy of Dermatology, American Society for Dermatologic Surgery and many more.
Under her direction, the Clinical Trials Division evaluates new and emerging technologies, devices, topical and oral medications, skin care products and prescription drugs and other therapies for the treatment and diagnosis of medical and cosmetic skin conditions. MDLR has a dedicated clinical research staff who is actively involved in multiple ongoing studies at any time. The results of these studies are published in peer-reviewed medical journals and presented at national and international meetings by the board-certified dermatologists of MDLR in Miami.
We are very proud that MDLR has been selected as a renowned and trusted clinical research facility by leading pharmaceutical and medical device companies worldwide. Our participation in research enables us to provide our patients with the most advanced care using the newest treatments and latest technologies available. The Clinical Trials Division consists of a team of core members who work to ensure that all clinical trials are conducted in accordance with standards of Good Clinical Practice.
Here at Miami Dermatology & Laser Research, we have a robust, unique clinical trial company. We offer innovative, cutting-edge research trials- in some cases patients receive treatment free of charge or even are paid for their participation. We draw from our extensive database to provide our patients with the opportunity to participate in some of the most ground-breaking drug and device trials. Although we do these treatments in house, we have no control over the treatment/visit schedule, payments, or changes to the study course- these are all determined and controlled by the sponsoring company, Investigational Review Board (IRB), and FDA. Miami Dermatology & Laser Research will communicate everything to the patient but is not responsible for the study details or the changing needs of the company which may alter the above items. We want our patients to continue to have outstanding service and care while being some of the first patients in the world to have access to new and exciting medical and cosmetic therapies.

